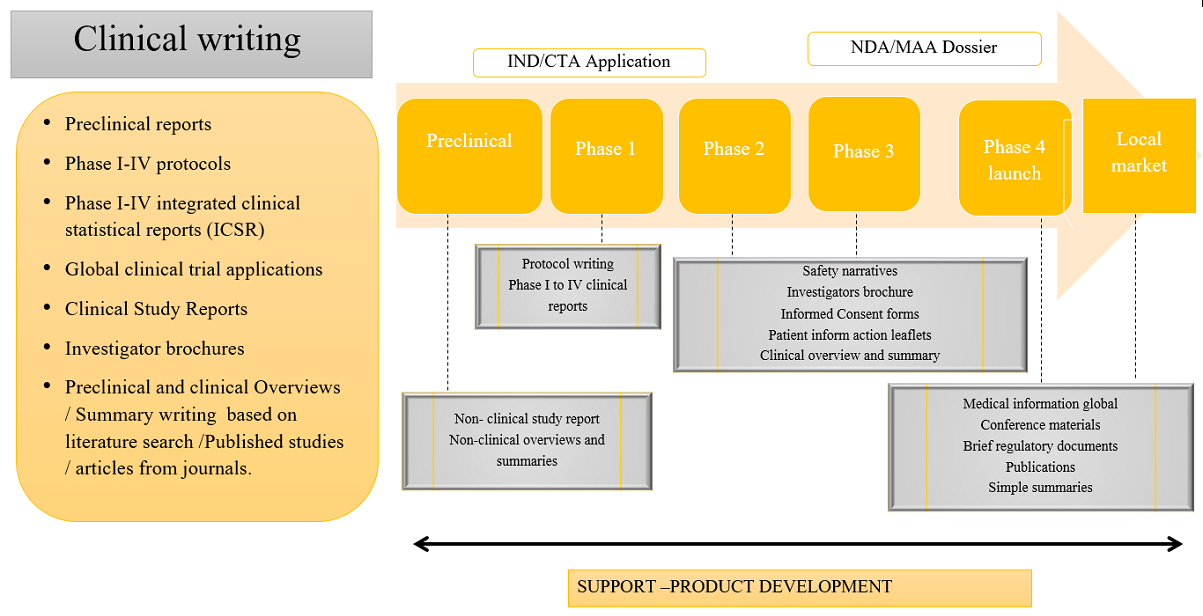
Investigators brochure (IB)
A comprehensive document prepared during a drug trial by summarizing the body of information about an Investigational product ("IP" or "study drug").
Clinical trial protocol
A clinical trial protocol describes how a clinical trial has to be conducted by ensuring utmost safety of study subjects and the integrity of collected data. A clinical trial protocol comprises the details of clinical trial such as the objective (s), design, methodology, statistical consideration and organization of clinical trial.
Case report forms (CRF)
A case report form (CRF) is a paper or electronic questionnaire used generally in clinical trial research. The case report forms serve as a tool to collect data including adverse events from each participating patient in clinical trial by the sponsor.